It was a dinner table conversation imprinted in my memory. The date: September 2021. Topic: Covid injections. Discussion: robust. With the mandatory vaccination deadline looming, I argued that mandates violated someone’s ability to give their consent because there was pressure, coercion, and manipulation. Also, people couldn’t give fully informed consent to a product that had been around for less than a year as the risks were not yet entirely known.
My colleague argued that the injections had been through rigorous safety testing, the risks were known, and any unknowns would be picked up by the TGA through their closely monitored Database of Adverse Events Notifications system. If there were concerns, we would be informed about them.
Maybe at an earlier time in my life, I would have felt similarly reassured. But you only need to understand a little of the history of medicine and the pharmaceutical industry to know that we are not good at picking up and acting on safety signals – think thalidomide, smoking, and any of a vast array of medications that have been removed from market sometimes decades after approval. Neither is the pharmaceutical industry a ‘model’ operator with some pretty serious convictions and criminal fines in recent history.
So, when the rollout started in 2021 I was scanning the horizon for concrete reassurance. I wasn’t the only one. Research analyst Sharon Cousins was watching closely too. Over the past three years Cousins, along with a team of volunteers, has been on a journey of data-discovery that has resulted in the creation of a startling resource for Australians.
She tells her story:
As a member of the public, I was concerned about the fast pace of the Covid vaccines being produced. Contacts of mine in the UK mentioned adverse reports being listed on the UK Government system called the ‘Yellow Card’ and I followed these and the reports in Israel and the USA because they started these trial vaccines ahead of us, before the rollout in February 2021 in Australia.
I was concerned when I saw a publicly-available report – the European Medicines Agency (EMA) Assessment report on the Pfizer product, Comirnaty. Because I’ve had breast cancer (twice), I wanted to see if the Pfizer Covid jab had been tested for cancer. The EMA Assessment Report on Comirnaty is a 140-page document dated the 19th of February 2021. Lo and behold, on page 55, it states: ‘No genotoxicity nor carcinogenicity (cancer-causing) studies have been provided.’ Then on page 115, I was very alarmed to see a whole table list called ‘Summary of Safety Concerns’.
The list Cousins found contained important identified risks – anaphylaxis, and important potential risks – this also included vaccine-associated enhanced disease, vaccine-enhanced respiratory disease, and a whole section called ‘missing information’. ‘Missing information’ included use in pregnancy, breastfeeding, immunocompromised patients, frail patients with co-morbidities, and patients with autoimmune or inflammatory disorders. The report also listed ‘long term safety data’ as ‘missing information’.
A similar list can be found on page 31 in the TGA’s Public Assessment Report dated January 2021 along with the decision, ‘Approved for provisional registration’.
Forgive me while I pause and ask the obvious.
If the safety information on our most vulnerable was missing at the time of the rollout, why wasn’t this made known to the public and the medical profession? Also, how can informed consent be possible if a patient is not made aware of these ‘known unknowns’?
Moving on.
In April 2021, with this information in hand, Cousins contacted the TGA’s Advisory Committee on Vaccines to voice her concerns. She is still waiting to hear back.
The TGA’s Covid-19 Vaccine Safety and Monitoring page makes clear the role of the TGA with regard to vaccines:
‘The Therapeutic Goods Administration (TGA) is responsible for monitoring the safety of all vaccines approved for use in Australia. We closely assess safety data prior to approval and continue to monitor the safety of vaccines after they are registered in Australia so that we can detect and respond to any safety concerns. This is known as pharmacovigilance.’
From the beginning of the Covid injection rollout in Feb 2021, the TGA began monitoring the adverse event reports through its Database of Adverse Events Notification (DAEN) system. The TGA describes DAEN – medicines as ‘our online database that contains information from suspected adverse events reported to us for medicines, vaccines and biological therapies’.
They continue:
‘We use information from these adverse event reports to detect unusual or unexpected patterns in the data which may indicate a safety signal related to a product. This passive signal detection surveillance system relies on people reporting an adverse event that they suspect is linked to a medicine.’
The TGA makes clear that ‘inclusion in DAEN – medicines does not mean that the adverse event has been confirmed or that it was caused by a medicine or vaccine’ and ‘there might be no relationship between the adverse event and the medicine. The symptoms may instead have occurred coincidentally’.
Regarding the reported deaths, the TGA states, in its final safety report on the Covid injections in November 2023, ‘The TGA has identified 14 reports where the cause of death was linked to vaccination from 1,004 reports received and reviewed (as of October 29, 2023). There have been no new vaccine-related deaths identified since 2022.’
Noted.
To date, there have been almost 140,000 adverse event reports entered to DAEN for the Covid injections, including 1,010 reported deaths.
At this point, you may be interested to know that the number of adverse events reported for the Covid injections amounts to around 20 per cent of all adverse events reported to the TGA – for all medicines combined – for the past 50 years.
Now, I’m no statistician but even if only a fraction of these reports are found to be ‘causal’ this looks like a safety signal to me, and I have questions.
Many have argued, ‘There have been millions of doses, and therefore you would expect a lot of reports.’ That may be so. However a ‘passive surveillance’ system is only as good as the reports that are entered, and a significant under-reporting factor needs to be considered and has been reported in the literature. Anecdotally, I have been told many accounts of people suffering a suspected adverse event only to be refused to have their concerns acknowledged by their doctor, let alone added to the DAEN as a report.
(It may encourage the reader to know that anyone – member of the public, health care professional, patient, or family member – can report a suspected adverse event to a therapeutic product here.)
Back to the DAEN. Anyone who has tried to navigate DAEN will have discovered it is not the most user-friendly interface. For example, it is difficult to find and identify demographics, patterns of adverse events, or even reported deaths. With less than 20 per cent appearing to have the batch number recorded it is also challenging to ascertain whether particular batches are associated with higher numbers of reports, as has been found overseas.
Enter the Freedom of Information request process.
‘I requested and paid for a TGA Freedom of Information document entitled FOI 4077,’ Cousins tells me. ‘It was published on the TGA FOI Disclosure Log website in December 2022. I asked for all the Covid vaccine TGA DAEN case numbers of reported deaths and their doses and batch numbers from the 10th of January 2022, which was when they started jabbing the 5–11-year-old children in Australia, up until the time of my request, which was November 8, 2022.’
What Cousins found alarmed her. Even with only a fraction of batch numbers provided, two batch numbers stood out – FL5333 associated with six reported deaths, and FP1430 associated with two reported deaths. By searching the DAEN system, Cousins found the case numbers for the two FP1430 recipients, two boys aged ten and five.
Cousins hasn’t been the only one requesting further information via the FOI process. There has been a virtual army of researchers requesting additional data from the TGA and the full FOI disclosure log can be found here.
This is information that – in my opinion – should be made more freely available to the public and the medical profession. There’s good news, it now IS!
Thanks to Cousins and a team of volunteers, the TGA’s entire, published, database of adverse event notifications for the Covid injections is now available on a user-friendly, easily searchable platform called OpenDAEN. Created through hundreds of hours of meticulous, painstaking work, each report has been entered and cross-matched with additional information obtained through the FOI process. Take a look:
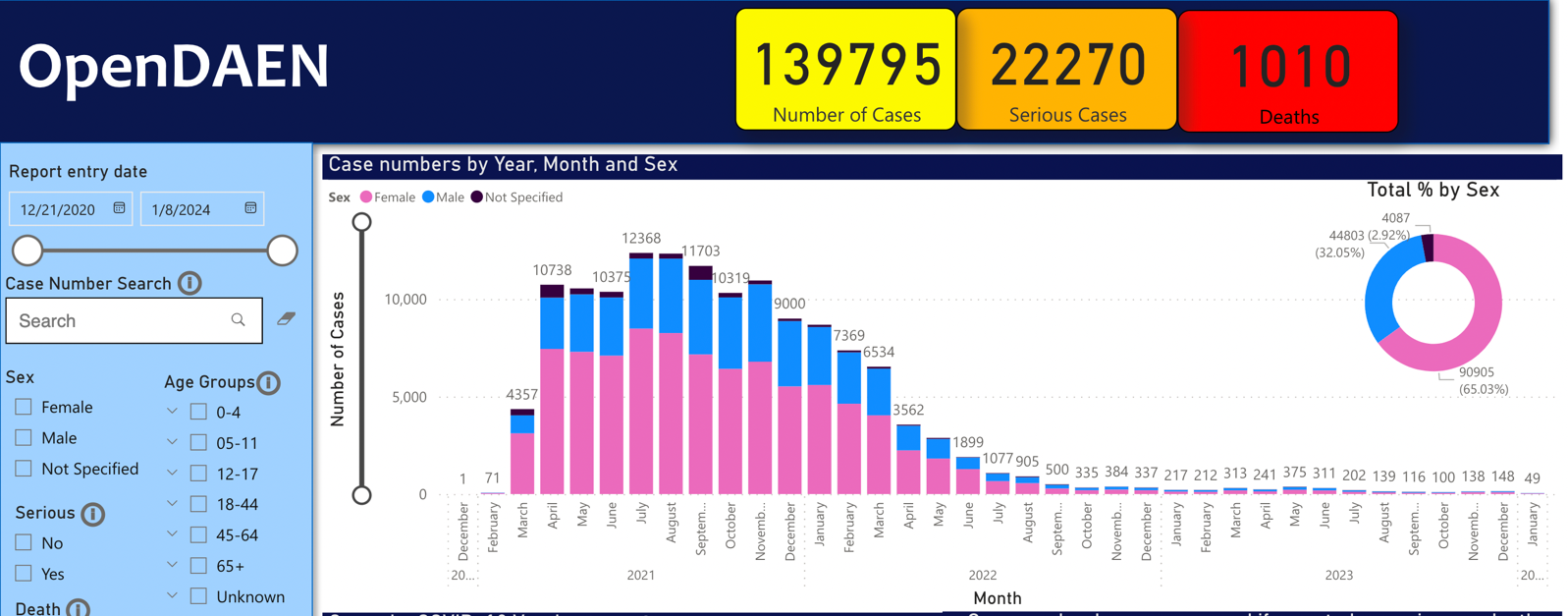
OpenDAEN allows for easy research of adverse event reports including serious cases, patterns of adverse events, product brand, and batch numbers where available. I have no doubt this invaluable resource will bring a fuller picture of Australian Covid adverse event reports, to the public, the medical profession, and the government. In addition, OpenDAEN, will help inform consent discussions like never before.